For decades, scientists have wrestled with a simple but profound puzzle: how did life’s genetic code first connect with proteins?
Today, all living cells rely on enzymes to attach amino acids to RNA in a process called aminoacylation. But those very enzymes are themselves built by ribosomes, which in turn need aminoacylated RNA to function. It’s a classic chicken-or-egg paradox at the heart of biology: which came first, the protein machines or the code that makes them?
A new study has now revealed a way out of this puzzle.
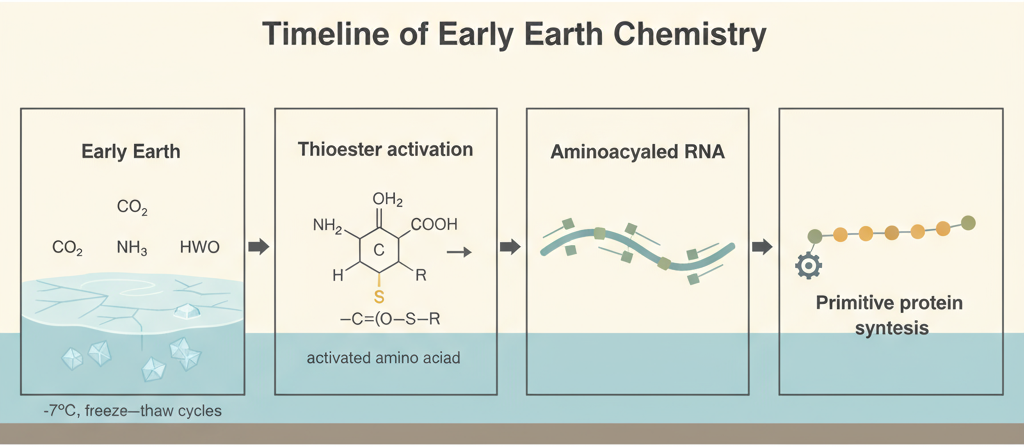
Aminoacylation: The first step toward proteins
In simple terms, aminoacylation means attaching an amino acid to RNA. This step is essential because it allows RNA to guide the assembly of proteins—the molecules that carry out most of life’s work.
But without enzymes, how could early life pull this off?
Thioesters: Ancient chemical helpers
The answer lies in chemistry older than biology itself. The researchers discovered that thioesters, sulfur-containing compounds likely abundant on the early Earth, could provide the necessary spark.
Thioesters activated amino acids, making them reactive enough to bond with RNA. In effect, these simple molecules acted as chemical stand-ins for enzymes, enabling a reaction that once seemed impossible without biological machinery.
Interestingly, thioesters still play a central role in modern metabolism, especially through coenzyme A, hinting that our own biochemistry carries echoes of this ancient chemistry.
A pathway in water—and in ice
Even more remarkable, this reaction worked in plain water—the very medium where life began. When conditions were cooled to around –7°C, the process became even more efficient.
This finding invites us to picture the icy ponds and shallow seas of the early Earth, where freeze-thaw cycles could have provided natural laboratories for these critical first steps toward life.
Breaking the paradox
This chemical pathway bypasses the paradox of life’s beginnings. It shows that RNA could be aminoacylated without the help of enzymes, long before proteins or ribosomes existed.
That means RNA could carry amino acids on its own, setting the stage for the eventual emergence of the ribosome—biology’s great protein-building factory.
Why it matters
By demonstrating that aminoacylation can happen without enzymes, this research bridges a crucial gap between RNA chemistry and protein chemistry. It suggests a realistic scenario for how life’s code began, dissolving one of the toughest paradoxes in origins-of-life science.
It’s a reminder that biology’s deepest mysteries often come down to chemistry—and that life’s beginnings may have been less miraculous and more inevitable than we once thought.
Conclusion
The discovery that thioesters can link amino acids to RNA without enzymes shows us how life may have started with simple, elegant chemistry. This isn’t just about ancient history—it’s about understanding the fundamental logic of life, and what truly defines it.
Life, it seems, began not with complex machines, but with molecules finding clever ways to copy, connect, and persist.
Suggested Reading
Singh, J., Thoma, B., Whitaker, D. et al. Thioester-mediated RNA aminoacylation and peptidyl-RNA synthesis in water. Nature 644, 933–944 (2025). https://doi.org/10.1038/s41586-025-09388-y







Leave a comment