Biochemistry
BiochemistryEvolution  Amazing Zoology2 Mins read
Amazing Zoology2 Mins read
Life before enzymes: How chemistry broke the paradox of life’s code
For decades, scientists have wrestled with a simple but profound puzzle: how did life’s genetic code first connect with proteins? Today, all living...
BiochemistryGeneral Zoology  Amazing Zoology1 Mins read
Amazing Zoology1 Mins read
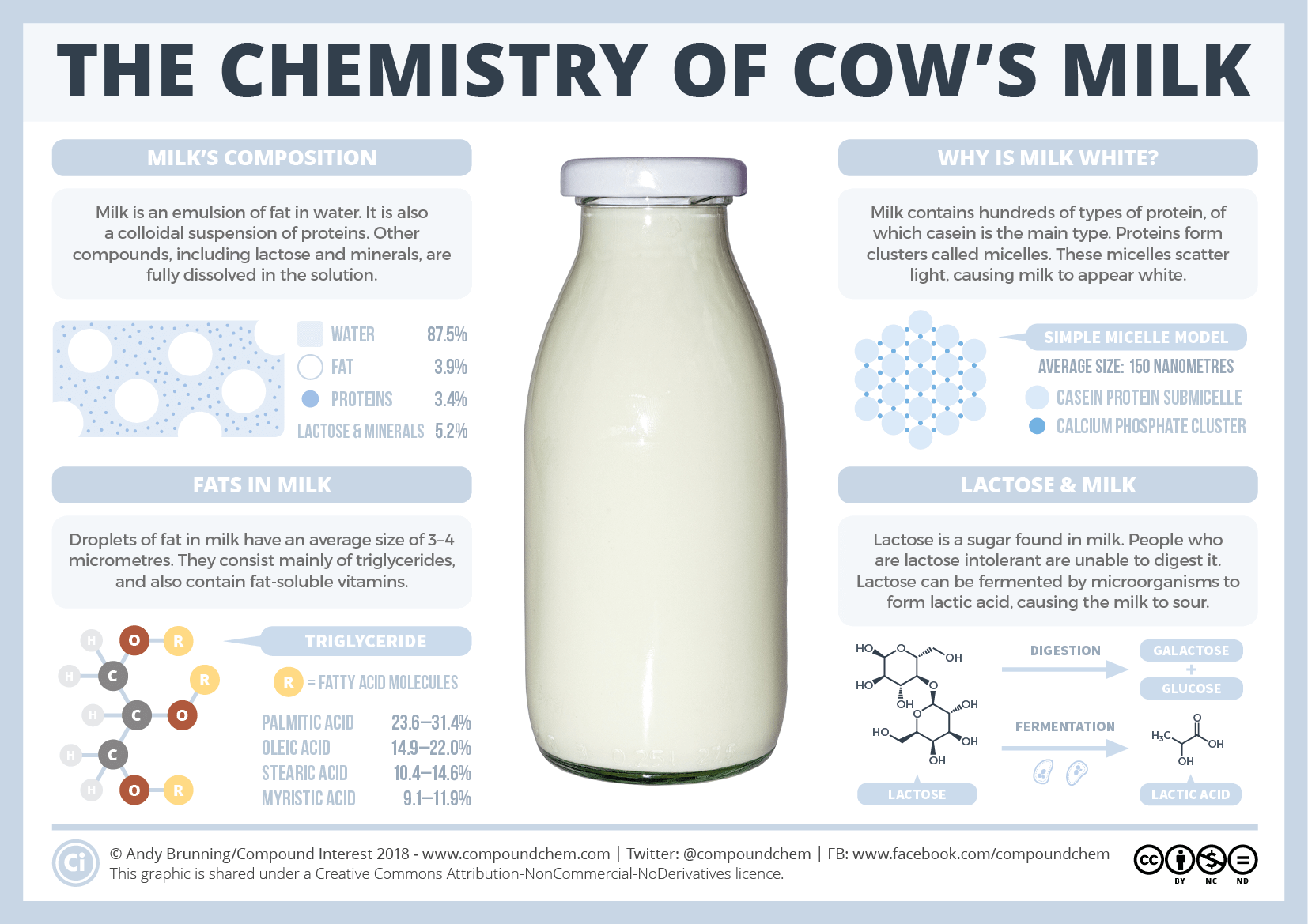
The chemistry of milk
In milk, proteins cluster together to form structures called micelles. They grow from small clusters of calcium phosphate, which held the proteins together....
BiochemistryEcologyEvolutionFeaturedPhysiology  Amazing Zoology2 Mins read
Amazing Zoology2 Mins read
What makes poison dart frog resistant to their own poison?
Poison dart frogs store Batrachotoxin, a steroidal alkaloid toxin in their skin glands. A single amino acid substitution is responsible for the resistance...
BiochemistryCell BiologyMolecular Biology  Amazing Zoology1 Mins read
Amazing Zoology1 Mins read
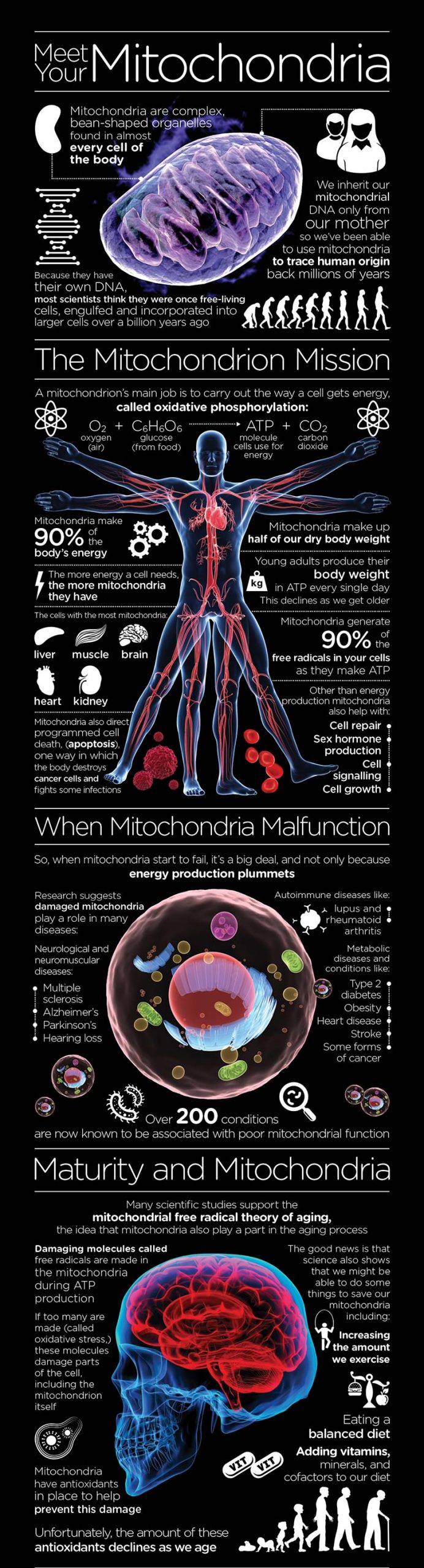
Know your Mitochondria – Infographic
© www.mitoq.com
BiochemistryPhysiology  Amazing Zoology2 Mins read
Amazing Zoology2 Mins read
Why does the consumption of alcohol produce a burning sensation?
Contents1 Why does drinking alcohol feels like burning?2 These were the old theories:3 Here is the truth!4 Whole thing can be summerised as5...
BiochemistryPhysiology  Amazing Zoology1 Mins read
Amazing Zoology1 Mins read
How do animals see in the dark?
We humans can’t do anything in dark as we can’t see in dark. But many animals like cats and owls can see in...